Abstract
Background: Multiple Myeloma (MM) is the second-most frequently diagnosed hematological malignancy. Although the incidence of MM in Asia is lower than that in Western countries, it is steadily increasing. Limited data are available on patterns of treatment use and the impact on survival from MM in Asia.
Objective: To describe patterns of treatment in patients aged ≥18 years with newly diagnosed MM in Taiwan, and to estimate the association with survival.
Methods: We conducted a retrospective cohort study utilizing the Taiwan National Healthcare Insurance Research database (NHIRD). The Taiwan NHIRD database is a population based database covering 99% of the population. All newly diagnosed MM patients in the catastrophic illness registry from 2007-2012 were enrolled. Patients with any pre-existing primary cancer other than MM were excluded. Eligible patients were followed up until death or end of the observation period (December 31, 2013), whichever occurred first.
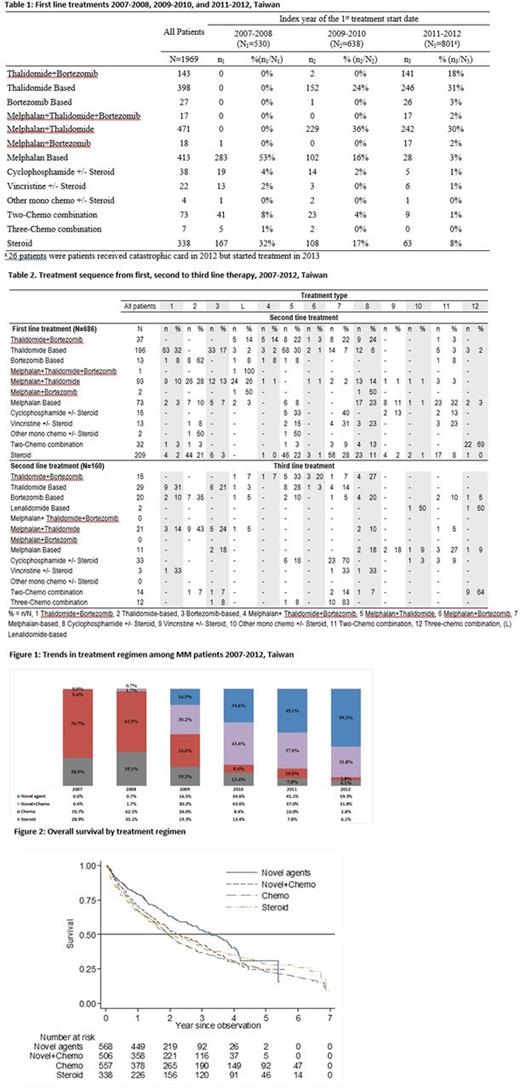
Results: The analysis included 1,969 patients with newly diagnosed MM, of whom 57.1% (1,125/1,969) were male. Treatment regimens changed over time corresponding to the introduction of novel agents in Taiwan (thalidomide in 2000, bortezomib in 2007): 70.7% received chemotherapy in 2007 versus 2.8% in 2012 (fig. 1). There were more patients <65 years who received treatment with novel agents (thalidomide + bortezomib, thalidomide-based or bortezomib-based) than patients >65. Patients >65 years mostly received melphalan-based and melphalan + thalidomide regimens.
The most frequently used first line treatments changed from melphalan-based chemotherapy or steroids in 2007-08, to thalidomide-based or thalidomide + melphalan in 2009-10, with addition of bortezomib in 2011-12 (approved for first line treatment in 2012) (Table 1). After first line treatment, 217/1969 (11.0%) patients received maintenance therapy (the same regimen recommenced after an interval of at least 60 days), 686 (34.8%) received second line treatment (Table 2), 18 (0.9%) were re-treated (first line agents re-prescribed after an interval of at least 180 days), 51 (2.6%) were treated for relapsed/refractory MM, 640 (32.5%) died, 61 (3.1%) underwent transplant, 46 (2.3%) received no treatment and 250 (12.7%) continued first line treatment until the end of the observation period. After second line treatment, 122/686 (17.8%) patients received second line maintenance therapy, 160 (23.3%) received third line treatment (Table 2), 6 (0.9%) were re-treated with second line agents, 21 (3.1%) were treated for relapsed/refractory MM, 163 (23.8%) died, 67 (9.8%) underwent transplant, 13 (1.9%) received no treatment and 134 (19.5%) were censored.
The mean treatment duration was 368 days (SD 407 days) for first line treatment and 267 days (SD 322) for second line treatment. The mean interval between cessation of first line treatment and commencement of second line treatment was 11 days (SD 65). The mean interval between second and third line treatment was 28 days (SD 65).
Survival decreased as age increased. Overall survival was higher in patients who received novel agents compared to other therapies (not adjusted for age) (fig. 2).
Conclusion: Data from a nationally representative database show evolving treatment patterns in Taiwan from 2007-2012, reflecting the introduction of novel agents for MM. Despite the introduction of new treatments in the last decade, MM remains largely incurable with high mortality.
Qiu: Janssen Research & Development: Employment, Other: holds shares in Janssen Research & Development . Siggins: Janssen Research & Development: Employment. Rothwell: Janssen Research & Development: Employment. Yang: Janssen Medical Affairs: Employment. Zhang: Janssen Research & Development: Employment. Liu: Janssen Research & Development: Employment, Other: hold shares in Janssen Research & Development .
Author notes
Asterisk with author names denotes non-ASH members.